Alevel化学课程是一度被评为最难的Alevel课程之一的一门,在IGCSE阶段,有很多同学对化学这门科目的学术性和实践性难度都有所了解。它的知识点数量多且细碎,有很多原理、概念和公式需要记忆,本篇中,我们考而思惟世为大家带来的是AS阶段的基础知识原子核结构的相关内容,一起来看看吧!

The nucleus 原子核
The protons and neutrons in each atom are tightly packed in a positively charged nucleus. Negatively charged electrons move around the nucleus. The number of protons in a nucleus defines the type of atom and is the same as the atomic number. The number of neutrons is found by subtracting the atomic number from the mass number. In an atom because there is no overall charge the number of electrons equals the number of protons.
每个原子中的质子和中子都紧紧地挤在一个带正电的原子核中。带负电的电子在原子核周围移动。原子核中的质子数决定了原子的类型,与原子数相同。中子的数量是通过从质量数中减去原子数而得到的。在一个原子中,由于没有整体电荷,电子数等于质子数。
In chemical reactions, the nucleus remains unchanged.
在化学反应中,原子核保持不变。
Geiger and Marsden bombarded a thin gold foil with a beam of alpha particles.
盖格和马斯登用一束α粒子轰击薄薄的金箔。
Most of the particles passed through the foil without deflection and were detected by a flash of light when the alpha particle struck a zinc sulphide screen, surrounding the gold foil.
大多数粒子在没有偏转的情况下通过了金箔,并在α粒子击中金箔周围的硫化锌屏幕时被一道闪光检测到。
A few were deflected and some of these were deflected at angles greater than 900, suggesting they had been repelled by large positive charges within the foil - nuclei of atoms of gold.
少数粒子被偏转,其中一些被偏转的角度大于900,这表明它们被金箔内的大型正电荷--金原子核--所排斥。
Arrangement of electrons around the nucleus 电子围绕原子核的排列方式
From GCSE you should be familiar with the Bohr model of electrons arranged around a nucleus. The electrons are in certain energy levels and each energy level can hold only up to a maximum number of electrons.
从GCSE开始,你应该熟悉电子围绕原子核排列的玻尔模型。电子处于一定的能级,每个能级只能容纳最多的电子数。
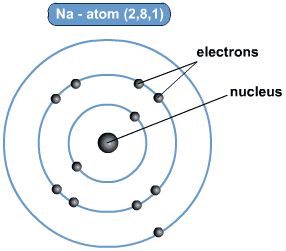
A sodium atom containing 11 electrons has an electron arrangement of 2,8,1. Two electrons filling the first shell, eight electrons filling the second shell and one electron in the outer third shell.
一个含有11个电子的钠原子的电子排列是2,8,1。两个电子填充第一层外壳,八个电子填充第二层外壳,一个电子在外面的第三层外壳。
However, these models of electron arrangement are simple and a more advance done can now be used. It is possible to break these energy levels into sub-shells.
然而,这些电子排列的模型是简单的,现在可以使用一个更先进的完成。有可能将这些能级分解成子壳。
Electrons are impossible to locate exactly at any one time. It is however, possible to indicate a region or volume where the electron is most likely to be found. This region is called an Orbital.
电子在任何时候都不可能被准确定位。然而,有可能指出电子最有可能被找到的一个区域或体积。这个区域被称为 "轨道"。
Each orbital is capable of holding a maximum of 2 electrons. Orbitals can be divided intos, p, d, and ftypes. Each type has its own characteristic shape.
每个轨道最多能够容纳2个电子。轨道可以分为s、p、d和f类型。每种类型都有自己的特征形状。
以上就是小编为大家整理的AS化学考试必考考点之一的总结,希望对大家有所帮助,祝学业有成金榜题名!如有更多有关Alevel辅导的需要,欢迎来联系在线客服老师,会获得更多专业的指导哦~
凡来源标注“惟世教育”均为惟世教育原创文章,版权均属惟世教育所有,任何媒体、网站或个人未经本网协议授权不得转载 链接、转贴或以其他任何方式复制、发表。未注明来源等稿件均为转载稿,如涉及版权请联系在线客服处理。
免费获得学习规划方案
已有 2563 位留学生获得学习规划方案



马上领取规划
*已对您的信息加密,保障信息安全。

 在线咨询
在线咨询
 免费通话
免费通话